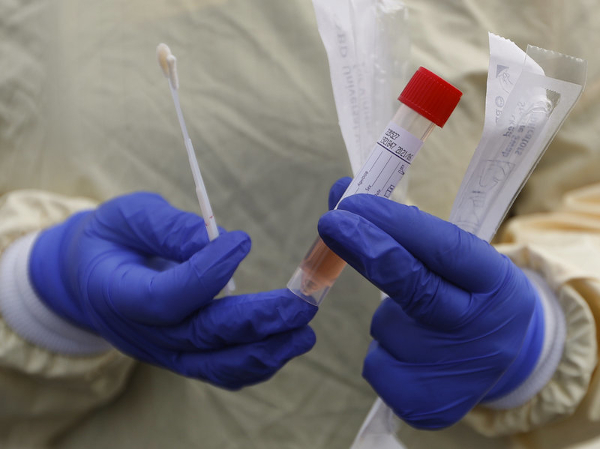
Technologists at the Sree Chitra Triunal Institute for Medical Sciences and Technology (SCTIMST), an autonomous institute under the Department of Science and Technology, Govt of India, have developed two types of nasal and oral swabs and viral transport medium for COVID-19 testing.
Chitra EmBed flocked nylon swabs (co-developed with Mallelil Industries Pvt ltd), and Chitra EnMesh, polymeric foam-tipped, lint-free swabs with flexible plastic handles developed by technologists Dr. Lynda V Thomas, Dr. Shyni Velayudhan and Dr. Maya Nandakumar from SCTIMST have both proven efficiency in the adequacy of specimen collection and rapid elution (extracting one material from another by washing with a solvent) of specimen into the liquid viral medium. They also have good recovery of viral RNA collected using these swabs and medium. The swabs will be available as sterile, ready-to-use devices.
The swabs are designed for efficiency and comfort in the working environment and help in improved specimen collection with minimum discomfort to patients. Their safe and convenient breakpoint ensures minimal contact of the health worker with the sample during packing.
The second innovation, Chitra Viral Transport Medium, is specifically designed to retain the virus in its active form during its transportation from the collection point to the laboratory. Currently, kits containing 50 (3ml/vial) viral transport medium with 50 swabs cost is upwards of Rs 12000/.
Technologies for both swabs and viral transport medium have been transferred to two industries for immediate manufacture and sales– Mallelil Industries, Origin diagnostics, and Levram Life sciences.
Currently, Nasal and throat specimens collected with specially designed swabs are used for the detection of SARS-COV2 by viral gene amplification method, which is necessary for the confirmation of COVID 19. Proper and adequate specimen collection and its transport in a suitable liquid medium are critical for ensuring good quality and quantity of viral RNA from the sample for testing, as these influence the accuracy of the test. Centre for disease control and prevention (CDC),the USA, recommends the use of synthetic fibre swabs with plastic shafts, preferably flocked swabs when available.
These two swabs developed with locally available material can reduce import dependency of the materials currently used and can meet the huge demand for them at much lower costs.
Source: PIB
Image Courtesy:NPR
You may also like
-
Dr Jitendra Singh Honours 26 Young Doctors, Highlights Role of AI and Specialisation in Future Healthcare
-
Chundanga (Turkey Berry): The Bitter Green Healer of the Tropics
-
India’s Transformation into a Global Health Powerhouse — A New Era in Public Health
-
India’s Historic HPV Vaccine Drive: Turning the Tide Against Cervical Cancer
-
Ministry of Ayush Inaugurates India’s First Integrative Oncology Research and Care Centre at AIIA Goa